A Theory of Heat Transfer Between Two Gases in a Mixture
Nuclear Science and Engineering / Volume 60 / Number 3 / July 1976 / Pages 311-314
Technical Note / dx.doi.org/10.13182/NSE76-A26887
Articles are hosted by Taylor and Francis Online.
Starting from the mechanics of collision between two perfectly elastic smooth spherical molecules, the following equation for the heat transfer rate per unit volume from a gas or vapor 2 to another gas 1 in a mixture is derived based on the kinetic theory of gases: 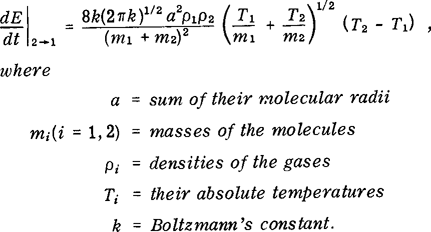 Methods of estimating molecular diameters when experimental values are not available are indicated, and values for sodium and UO2 vapor are estimated. For a set of typical values of the parameters, the time constant for the heat transfer is found to be of the order of 10−8 sec, which implies that for processes occurring in time periods greater than those of the order of 10−8 sec, the gases can be assumed to come to a thermal equilibrium at the instant they mix.
Methods of estimating molecular diameters when experimental values are not available are indicated, and values for sodium and UO2 vapor are estimated. For a set of typical values of the parameters, the time constant for the heat transfer is found to be of the order of 10−8 sec, which implies that for processes occurring in time periods greater than those of the order of 10−8 sec, the gases can be assumed to come to a thermal equilibrium at the instant they mix.


