First-Principles Study of Water Adsorption on 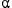 -Al2O3(0001): Influence of Hydrogen Isotope
-Al2O3(0001): Influence of Hydrogen Isotope
Fusion Science and Technology / Volume 60 / Number 3 / October 2011 / Pages 1155-1158
Blanket and Breeder Materials / Proceedings of the Ninth International Conference on Tritium Science and Technology / dx.doi.org/10.13182/FST11-A12620
Articles are hosted by Taylor and Francis Online.
Adsorption of H2O on the 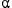 -Al2O3 (0001) surface was studied by means of a first-principles calculation based on density functional theory (DFT). We also investigated the behavior of the isotope exchange by substituting a protium atom with deuterium or tritium. The oxygen atom of H2O adsorbs on the Al atom of the outermost surface layer, the entire water molecule is slanted at the direction of a hollow site, and a molecular plane is nearly parallel to the surface. The adsorbed states are mostly due to coupling of lone-pair electrons of H2O with the empty p orbitals of the Al atom of surface. The behavior of dissociation for H2O is clarified from molecular dynamics simulations, indicating that the second neighbor oxygen atom is more preferable adsorption site for dissociation than the nearest neighbor oxygen atom on the surface.
-Al2O3 (0001) surface was studied by means of a first-principles calculation based on density functional theory (DFT). We also investigated the behavior of the isotope exchange by substituting a protium atom with deuterium or tritium. The oxygen atom of H2O adsorbs on the Al atom of the outermost surface layer, the entire water molecule is slanted at the direction of a hollow site, and a molecular plane is nearly parallel to the surface. The adsorbed states are mostly due to coupling of lone-pair electrons of H2O with the empty p orbitals of the Al atom of surface. The behavior of dissociation for H2O is clarified from molecular dynamics simulations, indicating that the second neighbor oxygen atom is more preferable adsorption site for dissociation than the nearest neighbor oxygen atom on the surface.


