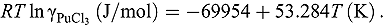Home / Publications / Journals / Nuclear Technology / Volume 206 / Number 4
Thermochemical Evaluation of Standard Electrode Potential and Gibbs Energy of Formation of PuCl3 in LiCl-KCl Eutectic Melt
Nuclear Technology / Volume 206 / Number 4 / April 2020 / Pages 587-608
Technical Paper / dx.doi.org/10.1080/00295450.2019.1666602
Articles are hosted by Taylor and Francis Online.
This paper deals with the estimation of  and
and 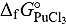 of the
of the 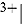
 value was also estimated in our laboratory at 723 and 773 K using logarithmic analysis of semi-integral voltammograms and chronopotentiograms. From semi-integral curves,
value was also estimated in our laboratory at 723 and 773 K using logarithmic analysis of semi-integral voltammograms and chronopotentiograms. From semi-integral curves, 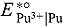 was found to be −2.888
was found to be −2.888 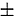 0.021 and −2.810
0.021 and −2.810  0.021 V at 723 and 773 K, respectively, whereas it was −2.851
0.021 V at 723 and 773 K, respectively, whereas it was −2.851 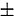 0.020 V at 773 K from the analysis of chronopotentiograms. Based on subsequent statistical analysis, we recommend the following expressions for
0.020 V at 773 K from the analysis of chronopotentiograms. Based on subsequent statistical analysis, we recommend the following expressions for 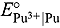
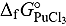 applicable for the temperature range 673 to 823 K:
applicable for the temperature range 673 to 823 K: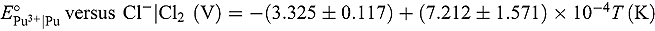 and
and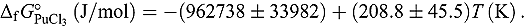
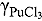 value in LiCl-KCl eutectic melt was estimated from
value in LiCl-KCl eutectic melt was estimated from 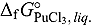 calculated for the reaction
calculated for the reaction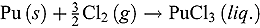 using FactSage package, and it was expressed as
using FactSage package, and it was expressed as