
Home / Publications / Journals / Nuclear Technology / Volume 68 / Number 3
Dissolution of Metal Oxides Accumulated in Nuclear Steam Generators: Study of Solutions Containing Organic Chelating Agents
Nuclear Technology / Volume 68 / Number 3 / March 1985 / Pages 385-394
Technical Paper / Material / dx.doi.org/10.13182/NT85-A33583
Articles are hosted by Taylor and Francis Online.
A study of the reactivity of ethylenediaminetetraacetic acid (EDTA), citric acid, and hydrazine for the dissolution of magnetite particles has allowed some steps of the different mechanisms to be identified. Two mechanisms are suggested: In acidic solutions, the chelating agents are adsorbed at the solid/solution interface followed by desorption of the complexed species FeHnL(n+1-4), where HnL is EDTA or citric acid, whereas in alkaline media, direct dissolution of the oxide particles takes place followed by complexation of the species Fe3+/Fe2+ in solution. The hydrazine apparently reduces the Fe3+ ions via a surface complexing reaction involving the 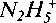 ions, a reaction which is in competition with the protonation of the Fe3O4 crystal lattice. Finally, regardless of the type of oxide (Fe3O4, Fe2O3, FeOOH, CuO, or Cu2O) or the composition of the complexing solutions, suspensions of these particles are highly unstable with respect to agglomeration or settling out, more because of the high concentration of chelating agents than their chemical characteristics.
ions, a reaction which is in competition with the protonation of the Fe3O4 crystal lattice. Finally, regardless of the type of oxide (Fe3O4, Fe2O3, FeOOH, CuO, or Cu2O) or the composition of the complexing solutions, suspensions of these particles are highly unstable with respect to agglomeration or settling out, more because of the high concentration of chelating agents than their chemical characteristics.

