
Home / Publications / Journals / Nuclear Technology / Volume 13 / Number 3
Electrochemical Measurement of the Solubility of Carbon in Sodium
Nuclear Technology / Volume 13 / Number 3 / March 1972 / Pages 289-296
Technical Paper / Material / dx.doi.org/10.13182/NT72-A31084
Articles are hosted by Taylor and Francis Online.
The solubility of carbon in sodium was measured with an electrochemical carbon meter by measuring the change in emf when known amounts of carbon were added to sodium contained in a nickel vessel. The solubility, Co, in ppm is given by the expression 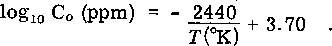 The data show little scatter, were easy to reproduce, and were not dependent on the rate of addition or the initial concentration of carbon. The results obtained are compared to the measurements reported in other investigations. The relation between the solubility data and the phenomenon of mass transport of carbon in sodium/stainless-steel systems is discussed. Oxygen was found to have no effect on the solubility of carbon in the concentration range from 1 to 240 ppm. When nitrogen is present at 1-atm pressure over the sodium it acts as a sink for carbon.
The data show little scatter, were easy to reproduce, and were not dependent on the rate of addition or the initial concentration of carbon. The results obtained are compared to the measurements reported in other investigations. The relation between the solubility data and the phenomenon of mass transport of carbon in sodium/stainless-steel systems is discussed. Oxygen was found to have no effect on the solubility of carbon in the concentration range from 1 to 240 ppm. When nitrogen is present at 1-atm pressure over the sodium it acts as a sink for carbon.

