
Home / Publications / Journals / Nuclear Technology / Volume 130 / Number 2
Measurements of the Effective Diffusion Coefficient of Dissolved Oxygen and Oxidation Rate of Pyrite by Dissolved Oxygen in Compacted Sodium Bentonite
Nuclear Technology / Volume 130 / Number 2 / May 2000 / Pages 206-217
Technical Paper / Radioactive Waste Management and Disposal / dx.doi.org/10.13182/NT00-A3088
Articles are hosted by Taylor and Francis Online.
The redox condition of the near field is expected to affect the performance of engineered barrier systems. In particular, the oxygen initially existing in the pore spaces of compacted bentonites strongly affects the redox condition of the near field. To assess the influence of the oxygen, research was done to assess its transport parameters in the compacted bentonite and consumption process. To understand the diffusion of dissolved oxygen (DO) in compacted bentonite and to predict the effect of the DO, the measurements of the effective diffusion coefficient of DO in compacted sodium bentonite were made by electrochemistry. As a result, the following relationship between the dry density of compacted sodium bentonite and the effective diffusion coefficient of DO in compacted sodium bentonite was derived: De = 3.0 ± 0.5 × 10-9 exp(-3.7 ± 0.2 × 10-3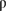 ), where De is the effective diffusion coefficient (m2 s-1) of DO in compacted sodium bentonite and
), where De is the effective diffusion coefficient (m2 s-1) of DO in compacted sodium bentonite and 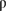 is the dry density (kg m-3) of compacted sodium bentonite.
is the dry density (kg m-3) of compacted sodium bentonite.
The oxygen concentration in the bentonite is expected to be controlled by the oxidation of pyrite as an impurity in the bentonite. To investigate this idea, the rates of pyrite oxidation by DO in compacted sodium bentonite were estimated from the experimental data in pyrite-bentonite systems using the obtained effective diffusion coefficient of DO. The results show that the average of the rate constants of pyrite oxidation by DO in compacted sodium bentonite was 1.16 ± 0.35 × 10-8 m s-1, whereas the rate constant in a carbonate-buffered solution (pH = 9.24) was 1.46 ± 0.09 × 10-9 m s-1.

