
Home / Publications / Journals / Nuclear Technology / Volume 40 / Number 2
The Interactions of Hydrazine, Ferrous Sulfamate, Sodium Nitrite, and Nitric Acid in Nuclear Fuel Processing Solutions
Nuclear Technology / Volume 40 / Number 2 / September 1978 / Pages 185-193
Technical Paper / Tutorial Materials/Design Interaction in Nuclear System / Chemical Processing / dx.doi.org/10.13182/NT78-A26714
Articles are hosted by Taylor and Francis Online.
Hydrazine and ferrous sulfamate are used as reductants in a variety of nuclear fuel processing solutions. An oxidant, normally sodium nitrite, must frequently be added to these nitric acid solutions before additional processing can proceed. The interactions of these four chemicals have been studied under a wide variety of conditions using a 2P factorial experimental design. It was determined that the desired oxidations of Fe2+, 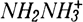 , and NH2SO3H to Fe3+ and N2 occur at ambient temperatures with nitric acid concentrations ≤3M without complicating side reactions. The rate of oxidation of Fe2+ by nitrous acid proceeds at about the same rate as the scavenging of nitrous acid by sulfamic acid. At nitric acid concentrations >3M and at elevated temperatures, hydrolysis of sulfamic acid to NH4HSO4 and decomposition of both hydrazine and nitrous acid become important side reactions.
, and NH2SO3H to Fe3+ and N2 occur at ambient temperatures with nitric acid concentrations ≤3M without complicating side reactions. The rate of oxidation of Fe2+ by nitrous acid proceeds at about the same rate as the scavenging of nitrous acid by sulfamic acid. At nitric acid concentrations >3M and at elevated temperatures, hydrolysis of sulfamic acid to NH4HSO4 and decomposition of both hydrazine and nitrous acid become important side reactions.

