
Home / Publications / Journals / Nuclear Technology / Volume 94 / Number 1
The Interaction of Iodine with Insoluble Residue in the Dissolution of Simulated Spent-Fuel Pellets
Nuclear Technology / Volume 94 / Number 1 / April 1991 / Pages 99-107
Technical Paper / Enrichment and Reprocessing / dx.doi.org/10.13182/NT91-A16225
Articles are hosted by Taylor and Francis Online.
To properly control radioiodine (129I) when reprocessing nuclear fuels, it is important to understand the interaction between iodine and the insoluble residue produced during the dissolution of spent fuels. Simulated spent-fuel pellets (∼1 g each) equivalent to spent fuel with a burnup of 5% fima were dissolved in 4.1 M HNO3 or a simulated spent-fuel solution to examine this interaction and the material balance of iodine. In dissolution in 4.1 M HNO3, 2 to 5% of the iodine in the pellet is conveyed to the insoluble residue (8 ± 1 mg), 1 to 5% remains in solution, and the balance volatilizes into the off-gas. The process that incorporates iodine into the residue is the formation of slightly soluble iodides, such as PdI2 and AgI, on the surface of the residue. The quantity of iodine in the residue averages 1.1 ± 0.5 µg I/mg of residue. Pellet dissolution in simulated spent-fuel solutions with a uranium concentration of ≧170 g U/ℓ and corresponding amounts of fission product elements causes a marked increase in the amount of residue and a significant increase in the amount of iodine involved. This phenomenon is due to the secondary precipitation of some metal molybdates. The PdI2 and AgI in the residue are in equilibrium with Pd2+, Ag+, and I- in the solution. The I- can be oxidized into I2 in a hot nitric acid solution bubbled with NO2. The action of NO2 causes part of the iodine in the residue to be eluted into the solution and then volatilized into the off-gas during the operation to expel iodine (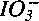 ) from the solution. A process consisting of (a) heating of the residue in a
) from the solution. A process consisting of (a) heating of the residue in a 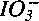 -concentrated HNO3 at 100°C and (b) introducing NO into the solution at 100°C will transfer 50 to 90% of the iodine in the residue to the gas phase. The remaining iodine is probably inside the residue as it is difficult to remove.
-concentrated HNO3 at 100°C and (b) introducing NO into the solution at 100°C will transfer 50 to 90% of the iodine in the residue to the gas phase. The remaining iodine is probably inside the residue as it is difficult to remove.

