Thermodynamics of Extraction of Nitric Acid by Tri-n-Butyl Phosphate—Hydrocarbon Diluent Solutions: III. Comparison of Literature Data
Nuclear Science and Engineering / Volume 14 / Number 2 / October 1962 / Pages 174-178
Technical Paper / dx.doi.org/10.13182/NSE62-A28117
Articles are hosted by Taylor and Francis Online.
Some of the published data on the extraction of nitric acid from aqueous solutions, containing ≦5 M acid, by tributyl phosphate-hydrocarbon diluent solutions were tested for their agreement with a mathematical description presented in Part 1. In all cases the agreement of the literature data with this description ranged from adequate to very good, thereby adding support to the interpretation of the parameters of the equation in terms of the equilibrium constant, 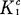 , for the extraction reaction and the two activity coefficients y′T and
, for the extraction reaction and the two activity coefficients y′T and 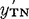 . The form of the mathematics suggests that TBP and TBP·H2O are indistinguishable in their reaction with HNO3 and that y′T is an approximate mean molar activity coefficient of these two in the water-saturated system; similarly,
. The form of the mathematics suggests that TBP and TBP·H2O are indistinguishable in their reaction with HNO3 and that y′T is an approximate mean molar activity coefficient of these two in the water-saturated system; similarly, 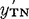 is an approximate mean molar activity coefficient of the two species TBP·HNO3 and TBP·HNO3H2O. The quantity
is an approximate mean molar activity coefficient of the two species TBP·HNO3 and TBP·HNO3H2O. The quantity 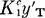 has a value of ∼1.5 in molar units for diluent-free TBP; its extrapolated value in the pure diluent Amsco 125-82, or odorless kerosene, is ∼0.23, while its value in n-hexane is ∼0.27.
has a value of ∼1.5 in molar units for diluent-free TBP; its extrapolated value in the pure diluent Amsco 125-82, or odorless kerosene, is ∼0.23, while its value in n-hexane is ∼0.27.


