Reaction Kinetics of Zirconium and Zircaloy-2 in Dry Air at Elevated Temperatures
Nuclear Science and Engineering / Volume 3 / Number 2 / February 1958 / Pages 171-185
Technical Paper / dx.doi.org/10.13182/NSE58-A25459
Articles are hosted by Taylor and Francis Online.
Corrosion rates of sponge zirconium and Zircaloy-2 in dry air were measured at 500, 600, and 700°C (930, 1110, and 1290°F). The reaction proceeds in two stages: initially the rate decreases with exposure time, approximating a cubic relationship; after sufficient exposure, the rate becomes a linear function of time. The rate constants calculated from the data and expressed by the Arrhenius equation, k = A exp (—Q/RT), are: 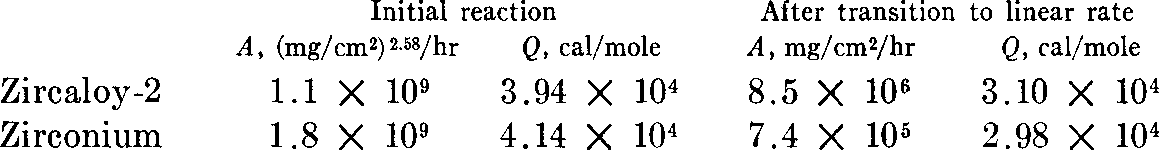 . Extrapolation of these data to lower temperatures shows that the service life of structures fabricated from these metals amounts to several years at temperatures below 400°C (750°F).
. Extrapolation of these data to lower temperatures shows that the service life of structures fabricated from these metals amounts to several years at temperatures below 400°C (750°F).


