Hydrolysis of Zinc Ion and Solubility of Zinc Oxide in High-Temperature Aqueous Systems
Nuclear Science and Engineering / Volume 127 / Number 3 / November 1997 / Pages 292-299
Technical Paper / dx.doi.org/10.13182/NSE97-03
Articles are hosted by Taylor and Francis Online.
Hydrolysis constants of the zinc ion were measured at 25, 50, 75, 185, 200, and 225°C through the direct measurement of pH using pH sensors, especially of the yttria-stabilized zirconia membrane-type in the case of high temperatures over 185°C, and evaluation was done on the temperature dependence of the hydrolysis constants of the zinc ion. Solubilities of zinc oxide in pure oxygenated water were measured at 150, 200, and 250°C. Equilibrium constants of zinc oxide dissolution and the values of 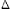 Gf0(Zn2+) at each temperature were estimated by thermodynamic analysis applying the estimated hydrolysis constants to the solubility data of ZnO.
Gf0(Zn2+) at each temperature were estimated by thermodynamic analysis applying the estimated hydrolysis constants to the solubility data of ZnO.


