Anomalous Reactions During Arcing Between Carbon Rods in Water
Fusion Science and Technology / Volume 26 / Number 3P1 / November 1994 / Pages 261-265
Technical Note / Nuclear Reaction in Solid / dx.doi.org/10.13182/FST94-A30330
Articles are hosted by Taylor and Francis Online.
Spectroscopically pure carbon rods were subjected to a carbon arc in highly purified water. The arc current varied from 20 to 25 A and was passed intermittently for several hours. The original carbon contained ∼2 parts per million (ppm) iron, and the detritus contained up to 286 ppm of iron. The carbon rods remained cool to the touch at >2 cm from their tips. Adsorption of iron from water or the surrounding atmosphere was established as not being the cause of the increase of iron. There is a weak correlation between the iron formed and the time of passage of current. When dissolved O2, was replaced by N2 in the solution, no iron was formed. Hence, the mechanism 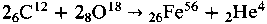 was suggested as the origin of the iron. The increase in temperature of the solution was consistent with expectation based on this reaction.
was suggested as the origin of the iron. The increase in temperature of the solution was consistent with expectation based on this reaction.


