Tritium Removal from Tritiated Water Using Mesoporous Silica
Fusion Science and Technology / Volume 60 / Number 4 / November 2011 / Pages 1395-1398
Detritiation and Isotope Separation / Proceedings of the Ninth International Conference on Tritium Science and Technology (Part 2) / dx.doi.org/10.13182/FST11-A12691
Articles are hosted by Taylor and Francis Online.
The ability of various solid adsorbents to adsorb tritium from tritiated water was studied. The tritium removability and adsorption ability of mesoporous silica (MCM-41) were found to be larger than those of conventional microporous zeolites such as mordenite (MOR) and Linde-type A (LTA). The different adsorbents can be arranged in order of tritium removability and tritium adsorption ability as follows: MCM-41 > LTA(5A) > high-silica MOR [approximately equal] low-silica MOR [approximately equal] LTA(4A). The adsorbents can also be arranged in decreasing order of the separation factor (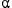 ) as follows: MCM-41 > LTA(5A) > low-silica MOR [approximately equal] LTA(4A) > high-silica MOR.
) as follows: MCM-41 > LTA(5A) > low-silica MOR [approximately equal] LTA(4A) > high-silica MOR.


